Known as, "The most abundant element in the universe", hydrogen is also the most abundant element on earth. Hydrogen is a very social element that seems to always by clinging to other elements, compounds and atoms such as  oxygen in water (H2O) or carbon in methane (CH4). Not surprising, it is also the most abundant element in all fossil fuels, i.e. Gasoline: CH3-(CH2)6-CH3, Diesel: C13H28, Oil: C8H18, Coal: CH. Even though coal is made up of hundreds of chemicals there is one hydrogen atom for every carbon atom in coal - the chemical equation of coal is simplified by just the C (carbon) and the H (hydrogen) symbols. In all cases hydrogen must be separated into pure hydrogen (H2) for use in fuel cell electric vehicles and used as a fuel in combustion applications. Hydrogen fuel combines with oxygen from the air through a fuel cell, creating electricity and water through an electro-chemical process that eventually sends the electrical current to the motors in a HFCV. FYI - hydrogen is also the most dominant element in the chemical structure of most drugs - Ibuprofen: C13H18O2.
oxygen in water (H2O) or carbon in methane (CH4). Not surprising, it is also the most abundant element in all fossil fuels, i.e. Gasoline: CH3-(CH2)6-CH3, Diesel: C13H28, Oil: C8H18, Coal: CH. Even though coal is made up of hundreds of chemicals there is one hydrogen atom for every carbon atom in coal - the chemical equation of coal is simplified by just the C (carbon) and the H (hydrogen) symbols. In all cases hydrogen must be separated into pure hydrogen (H2) for use in fuel cell electric vehicles and used as a fuel in combustion applications. Hydrogen fuel combines with oxygen from the air through a fuel cell, creating electricity and water through an electro-chemical process that eventually sends the electrical current to the motors in a HFCV. FYI - hydrogen is also the most dominant element in the chemical structure of most drugs - Ibuprofen: C13H18O2.
Production Processes
Hydrogen can be produced from several different resources including fossil fuels, biomass, coal and water. There are several ways to produce hydrogen:
-
SMR - Steam Methane Reforming of Natural: Synthesis gas—a mixture of hydrogen, carbon monoxide, and a small amount of carbon dioxide—is created by reacting natural gas with high-
 temperature steam. The carbon monoxide is reacted with water to produce additional hydrogen. This method is the cheapest, most efficient, and most common. Natural gas reforming using steam accounts for the majority of hydrogen produced in the United States annually.
temperature steam. The carbon monoxide is reacted with water to produce additional hydrogen. This method is the cheapest, most efficient, and most common. Natural gas reforming using steam accounts for the majority of hydrogen produced in the United States annually. -
Gasification: A synthesis gas can also be created by reacting coal or biomass with high-temperature steam and oxygen in a pressurized gasifier. This converts the coal or biomass into gaseous components. The resulting synthesis gas contains hydrogen and carbon monoxide, which is reacted with steam to separate the hydrogen.
-
Electrolysis: An electric current splits water into hydrogen and oxygen. If the electricity is produced by renewable sources, such as solar or wind, the resulting hydrogen will be considered renewable as well, and has numerous emissions benefits. Power-to-hydrogen projects are taking off, using excess renewable electricity, when available, to make hydrogen through electrolysis.
-
Liquid Reforming: hydrogen based liquid fuels, such as Hythanol®, are reacted with high-temperature steam to produce hydrogen near the point of end use.
-
Fermentation: Biomass is converted into sugar-rich feed stocks that can be fermented to produce hydrogen.
Each one of these processes have their own inherent dynamics, efficiency levels and yields per unit measured. Each process has its own electrical input requirement per unit of hydrogen (kilogram/cubic foot) as well as water consumption rate 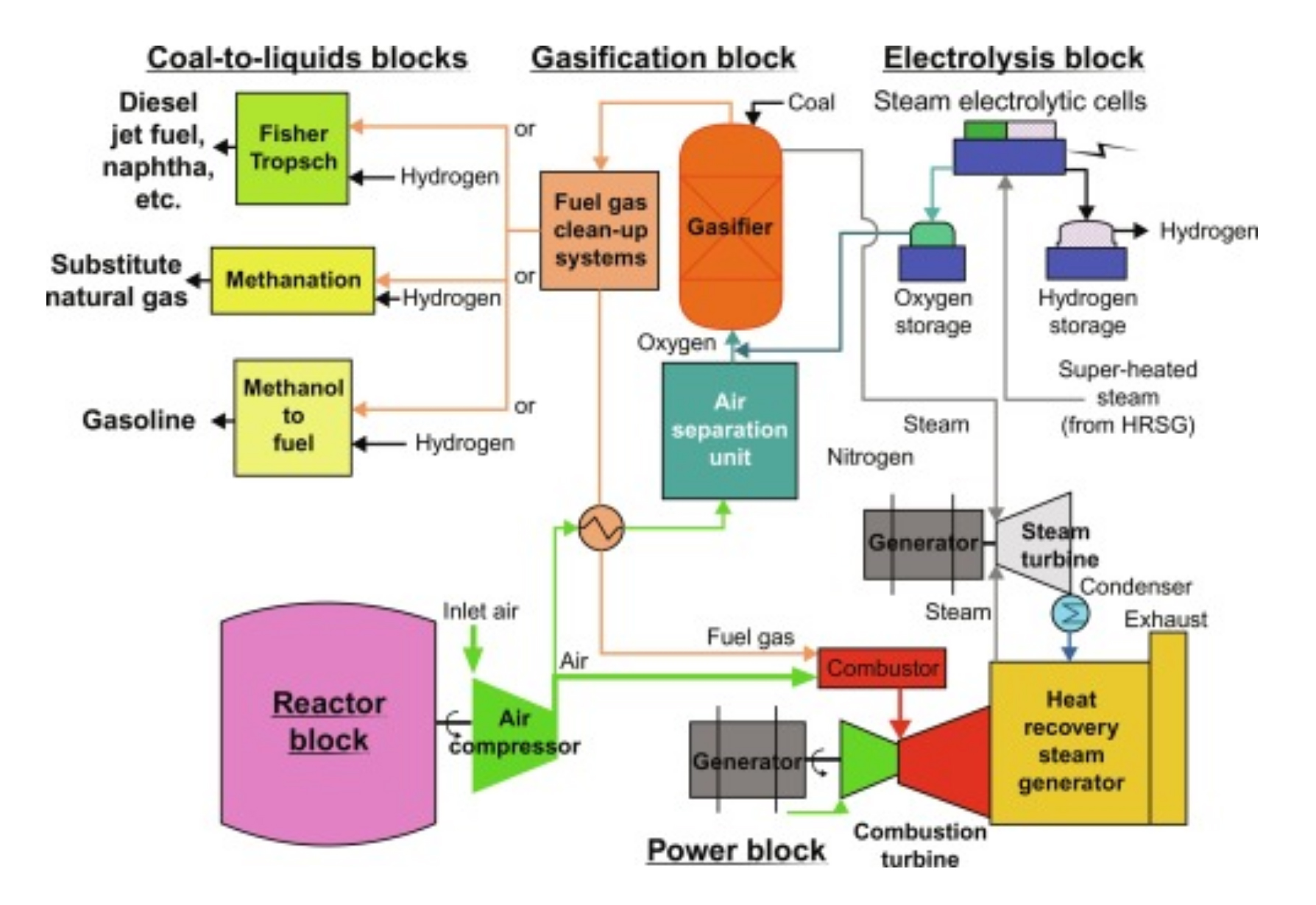 (gallon/liter per kilo) during the hydrogen extraction or separation of the molecular structure of each resource such as H2 being separated from O during electrolysis of water (H2O). Hydrogen via SMR of natural gas is by far the most economical process of producing hydrogen. But like all chemical and electrical processes - pricing varies by the fluctuating costs of the feed stocks: oil, gas, coal, natural gas, water and power rates.
(gallon/liter per kilo) during the hydrogen extraction or separation of the molecular structure of each resource such as H2 being separated from O during electrolysis of water (H2O). Hydrogen via SMR of natural gas is by far the most economical process of producing hydrogen. But like all chemical and electrical processes - pricing varies by the fluctuating costs of the feed stocks: oil, gas, coal, natural gas, water and power rates.
Hydrogen opponents zero in on the efficiencies of producing hydrogen, compressing, transporting and storing hydrogen via the different methodologies. This includes a long laundry list created by hydrogen opponents that also includes the emissions created in the production of hydrogen. Hydrogen opponents claim the high ground with the higher efficiencies during charging of BEVs and operation of the electrical drive train compared to that of HFCVs. But this claim to fame is short lived when you examine the entire value chain BEFORE one electron is sent vibrating along a high voltage transmission line that was created by a CO2 spewing power plant miles away that powers the grid that is connected to the charging systems for the batteries of EVs as was shown in the previous write up on "BEV's dirty little secret".
Electrolysis
Planet earth is covered by near 71% water. Like all fossil fuels - hydrogen is the most abundant element in one molecule of water (H2O). There are two atoms of hydrogen for every one atom of oxygen. there is a covalent bond (electromagnetic)  keeping the two hydrogen atoms attached to the oxygen atom. This free energy bond (like magnets) has an electrical value of about 110 kcal per mole (kilocalories). A mole (6.02 x 1023) is a unit counting number like dozen (12), gross (144), ream (500), inches in a foot (12) etc. used to count objects such as eggs, pencils, sheets of paper or rulers. To simplify the math we will look at the standard measurement of hydrogen when it comes to the production of hydrogen quantities which is "per kilo" or in some cases, "cubic meters per hour". There are 11.126 cubic meters in one kilo of hydrogen. I'm not certain why some manufacturers choose to use this latter measurement because hydrogen is sold by the kilo and hydrogen offtakers purchase by the kilo and HFCV owners pay by the kilo at the pump.
keeping the two hydrogen atoms attached to the oxygen atom. This free energy bond (like magnets) has an electrical value of about 110 kcal per mole (kilocalories). A mole (6.02 x 1023) is a unit counting number like dozen (12), gross (144), ream (500), inches in a foot (12) etc. used to count objects such as eggs, pencils, sheets of paper or rulers. To simplify the math we will look at the standard measurement of hydrogen when it comes to the production of hydrogen quantities which is "per kilo" or in some cases, "cubic meters per hour". There are 11.126 cubic meters in one kilo of hydrogen. I'm not certain why some manufacturers choose to use this latter measurement because hydrogen is sold by the kilo and hydrogen offtakers purchase by the kilo and HFCV owners pay by the kilo at the pump.
The standard enthalpy (remember I mentioned, "free energy") of formation of water is -286kJ/mol. Which means 286kJ of energy is produced when oxygen gas and hydrogen gas reacted to form 1 mole of liquid water. To convert that liquid into its gaseous components we need 241.8 kJ to break a molecule of liquid water and form two gaseous components of H2 and O2. Any method(thermal,electrolysis etc) followed, this much of energy will be always required. When using electrolysis, it may be convenient to express it in eV (electron-Volt). Ideally, it takes minimum 1.23 volts to split water molecules. Less voltage than this will give essentially no current, no electrolysis. However, over 1.8 V is needed in practice to overcome the activation barrier of the reaction.
The math of water becomes quite complicated from here on out but we will simplify. The density of water is 1g/(cm3) so in 1 gallon of water ( about 3.785 Liters or 3785 cm3) the mass of the water is, 3785g. 1 mole of 6.02x1023 molecules  of water is equal has the mass in grams equal to the molecular weight or 18 grams per mole. So 3785 grams corresponds to about 1.265 x 1026 molecules of water. Simplified - 18g of H2O will give 2g of H2 gas so for 1kg of H2 you will need 9000g or 9kg of water. There are 2.2 lbs per kilo - 9/2.2 = 4.09 lbs. The weight of water per gallon is 8.34 lbs - 8.34/4.09 = 2.04 gallons of water needed to produce one kilo of hydrogen via electrolysis. Due to electrical and friction looses the industry claims 2.6 gallons of water per kilo of hydrogen recovered via electrolysis. A typical containerized 1000 kilo per day electroysis system will consume 2,600 gallons of water per day - 949,000 gallons per year (2.91 acre foot annually).
of water is equal has the mass in grams equal to the molecular weight or 18 grams per mole. So 3785 grams corresponds to about 1.265 x 1026 molecules of water. Simplified - 18g of H2O will give 2g of H2 gas so for 1kg of H2 you will need 9000g or 9kg of water. There are 2.2 lbs per kilo - 9/2.2 = 4.09 lbs. The weight of water per gallon is 8.34 lbs - 8.34/4.09 = 2.04 gallons of water needed to produce one kilo of hydrogen via electrolysis. Due to electrical and friction looses the industry claims 2.6 gallons of water per kilo of hydrogen recovered via electrolysis. A typical containerized 1000 kilo per day electroysis system will consume 2,600 gallons of water per day - 949,000 gallons per year (2.91 acre foot annually).
This containerized electrolysis system will be delivered with a 2.5 MW power requirement and produce 492 Nm3/h configuration. Such a platform allows multiple units to be integrated easily in the field, which is a key advantage in scaling up with market demand.
I mentioned earlier how some companies use "Nm3/h" as a unit measurement where as hydrogen is sold and consumed by the kilo. The "492 Nm3/h" rating of this electrolyzer translates into - 492 X 24 = 11,808 Nm3 (Normal Cubic Meter). There are 11.126 cubic meters in one kilo of hydrogen - 11,808/11.126 = 1,061 kilos per day production. Since this unit is requiring 2.5 MW (60,000 kWhs per day) of power to produce that 1,061 kilos it equates to 56.55 kWhs per kilo. The common industry baseline of electrolysis ranges from 40 kWhs at the low end and 65 kWhs at the higher end.
Steam Methane Reforming (SMR)
According to the DOE, "Most hydrogen produced today in the United States is made via steam-methane reforming, a mature production process in which high-temperature  steam (700°C–1,000°C) is used to produce hydrogen from a methane source, such as natural gas. In steam-methane reforming, methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the presence of a catalyst to produce hydrogen, carbon monoxide, and a relatively small amount of carbon dioxide. Steam reforming is endothermic—that is, heat must be supplied to the process for the reaction to proceed."
steam (700°C–1,000°C) is used to produce hydrogen from a methane source, such as natural gas. In steam-methane reforming, methane reacts with steam under 3–25 bar pressure (1 bar = 14.5 psi) in the presence of a catalyst to produce hydrogen, carbon monoxide, and a relatively small amount of carbon dioxide. Steam reforming is endothermic—that is, heat must be supplied to the process for the reaction to proceed."
- CH4 + H2O + heat ⇌ CO + 3H2
- CO + H2O ⇌ CO2 + H2 + heat
The reformed gas is cooled and routed to a shift reactor to maximize the hydrogen content. The produced syngas is further cooled and process condensate is separated out. The reformed gas has an approximate composition of H2 74 mol%, CH4 7 mol%, CO 1 mol%, and CO2 18 mol%, the exact proportions depending on feed composition, operating conditions, and the selected process scheme. The median CO2 emission normalized for SMR hydrogen production was 9 kg CO2/kg H2 production, or 75 g CO2/MJ H2 (using H2 low heating value [LHV]).
Being a mature production process in which an external heat source provides high-temperature steam for the reforming reaction that produces hydrogen and CO2 from a gas source, such as methane. Any excess steam can be used to generate power, which is sufficient to meet the power demand of the overall plant. This assumes that standard SMR plants achieve a conversion efficiency of 74% (HHV). There are two main sources of CO2 emissions; one source is the CO2 that is produced alongside hydrogen in the reforming reaction, with the other being the CO2 produced by the external heat source that provides the high-temperature steam for the reaction. The process of capturing CO2 is far simpler for the former, with the capture of CO2 from fuel combustion being relatively expensive, as it needs to be separated from nitrogen. Overall, 90% of all CO2 is assumed to be captured.
According to a pricing report by S&P, "In the US market, and to a lesser extent in Europe, comparatively low feedstock cost for natural gas will likely make the SMR method of producing hydrogen more viable, at least in the near term.
Platts Analytics state, "SMR hydrogen can be produced at a cost under $1/kg, even assuming a natural gas price at $3.50/MMBtu. (Pricing sits at $4.50 per MMMBTU 2022) Adding carbon capture to make blue hydrogen raises the cost to roughly $1.40/kg. ($1.72 in 2022 dollars) Making the fuel green through a PEM (proton exchange membrane) electrolysis production method, though, more than triples that cost to an estimated $4.42/kg – assuming a renewable power cost of $65/MWh." This price $65 per MWh is more than double the going kWh rate.
Compression
Hydrogen is typically produced at relatively low pressures (20–30 bar) and must be compressed prior to transport. The energy required to compress a gas is a logarithmic function of the pressure ratio. The incremental energy input becomes smaller as higher pressures are reached. Multistage compression and intercooling are used to achieve high pressures. Most compressors used today for gaseous hydrogen compression are either  positive displacement compressors or centrifugal compressors. Positive displacement compressors can be reciprocating or rotary. Diaphragm compressors are the preferred choice for high purity and leak tight gas compression applications. Diaphragm compressors range in size from 3 hp(2 kW) to 200 hp(150kW). Discharge pressures range from 50 psi to as high as 15,000 psi (1,100 bar) and typical flow rates based on compression ratio to 500 Nm3/hr.(1090 kilos per day). This system is powered by a 150 KW motor running 24/7. It will consume 3,600 kwhs per day. If we combine the electrolysis power consumption of 60,000 kWhs per day per 1000 kilos plus the compression power requirements hydrogen production and compressions totals 63,600 kWhs - or a .05% increase in the cost of hydrogen. If we use a commercial grid pricing of $0.05 per kWh the cost of one kilo via electrolysis is $2.82 per kilo. Factoring in the kWh cost of compression one kilo per 1000 kilos per day requires 3.6 kwhs per kilo. Multiplying the same $0.05 per kWh compression adds $0.18 per kilo - Total: $3.00. We've now used a total of 60.15 kWhs to produce 1 kilo of hydrogen.
positive displacement compressors or centrifugal compressors. Positive displacement compressors can be reciprocating or rotary. Diaphragm compressors are the preferred choice for high purity and leak tight gas compression applications. Diaphragm compressors range in size from 3 hp(2 kW) to 200 hp(150kW). Discharge pressures range from 50 psi to as high as 15,000 psi (1,100 bar) and typical flow rates based on compression ratio to 500 Nm3/hr.(1090 kilos per day). This system is powered by a 150 KW motor running 24/7. It will consume 3,600 kwhs per day. If we combine the electrolysis power consumption of 60,000 kWhs per day per 1000 kilos plus the compression power requirements hydrogen production and compressions totals 63,600 kWhs - or a .05% increase in the cost of hydrogen. If we use a commercial grid pricing of $0.05 per kWh the cost of one kilo via electrolysis is $2.82 per kilo. Factoring in the kWh cost of compression one kilo per 1000 kilos per day requires 3.6 kwhs per kilo. Multiplying the same $0.05 per kWh compression adds $0.18 per kilo - Total: $3.00. We've now used a total of 60.15 kWhs to produce 1 kilo of hydrogen.
Since this system will compress hydrogen at 1000 bar (14,500 psi) - way beyond the 700 bar (10,000 psi) needed for HFCVs - we can calculate the entire cost of compression from atmospheric hydrogen produced using electrolysis and then compressing that same hydrogen gas in preparation for compressed tube trucks for transportation at 350 bar compression at production facility to distribution.
Liquefaction of Hydrogen
Gaseous hydrogen is liquefied (cryogenic hydrogen) by cooling it to below −253°C (−423°F). Once hydrogen is liquefied it can be stored at the liquefaction plant in large insulated tanks. It takes energy to liquefy hydrogen —using today's technology, liquefaction consumes more than 30% of the energy content of the hydrogen. In addition, some amount of stored hydrogen will be lost through evaporation, or "boil off" of liquefied hydrogen, especially when using small tanks with large surface-to-volume ratios. Research to improve liquefaction technology, as well as improved economies of scale, could help lower the energy required and the cost. Over long distances, trucking liquid hydrogen is more economical than trucking gaseous hydrogen because a liquid tanker truck can hold a much larger mass of hydrogen than a gaseous tube trailer can. Challenges with liquid transportation include the potential for boil-off during delivery.
—using today's technology, liquefaction consumes more than 30% of the energy content of the hydrogen. In addition, some amount of stored hydrogen will be lost through evaporation, or "boil off" of liquefied hydrogen, especially when using small tanks with large surface-to-volume ratios. Research to improve liquefaction technology, as well as improved economies of scale, could help lower the energy required and the cost. Over long distances, trucking liquid hydrogen is more economical than trucking gaseous hydrogen because a liquid tanker truck can hold a much larger mass of hydrogen than a gaseous tube trailer can. Challenges with liquid transportation include the potential for boil-off during delivery.
Current hydrogen liquefaction plants operate with a relatively modest thermodynamic energy efficiency of 30 to 35%. There is great scope to increase the thermodynamic energy efficiency and reduce the total work input of the compression system to lower hydrogen liquefaction costs. Costs can be significantly lowered if the electrical power required by the liquefaction plant is provided from an inexpensive source such as a gas or a steam turbine. There is a lot of work being performed and sponsored by the DOE to improve the efficiencies of liquefaction to 50% using singular and double turbo expanders that can then be redirected back to the front end for compression of gaseous hydrogen and converting it to cryogenic (liquid) hydrogen.
The liquefaction cost was also shown to reach a value of $0.63/kg for the optimized large-scale type plant at a production rate of 30,000 kg/h when the cost of electricity is $0.04/(kW h). The advantages of liquefied hydrogen carriers is the volume of liquefied hydrogen is 1/800 that of gaseous hydrogen. Therefore, liquefied hydrogen is suitable for transporting a large amount of hydrogen with high efficiency. Liquefied hydrogen has a purity of 99.999% or higher and can be directly supplied to fuel cells only by evaporating it without refining, which needs domestic energy. Hydrogen is also not toxic and has no greenhouse effect; thus, an excellent substance in terms of health and safety. The scheme of liquid hydrogen supply chain is like that of the supply chain of liquefied natural gas (LNG). LNG, which is currently distributed all over the world, was once very expensive. However, as years pass and the volume of distribution increases, it has become relatively affordable energy. Therefore, hydrogen can potentially and significantly decrease the cost in the future. Those are the reasons we chose liquid hydrogen as a carrier. The advantages of the higher costs of liquefaction outweighs the lower cost of compression due to the fact cryogenic trailers are able to transport double and triple the amount of hydrogen to points of distribution.
Hydrogen Transportation
We've looked at the increasing costs of hydrogen production via electrolysis Tube trailers are currently limited to pressures of 250 bar by U.S. Department of Transportation (DOT) regulations,  but exemptions have been granted to
but exemptions have been granted to  enable operation at higher pressures (e.g., 500 bar or higher). Steel tube trailers are most commonly employed and carry approximately 380 kg onboard; their carrying capacity is limited by the weight of the steel tubes. Recently, composite storage vessels have been developed that have capacities of 560–900 kg of hydrogen per trailer. These Chart LH2 tractor trailer tankers have a gross capacity of 17,600 gal / 66,623 liters, a maximum permissible weight of Liquid at 9,665 lb / 4,384 kg of LH2 and and estimated weight full (Capacity) 62,095 lb / 28,165 kg. It becomes obvious a expansive difference between delivery yields of hydrogen in cryogenic form compared to highly compressed tube trailers at only 350 kilos. The generated revenue of hydrogen to distribution between compressed hydrogen and LH2 tells a realistic story of profitability depending on hypothetical wholesale purchase agreements based on present day retail price at the pump of $16.50, i.e. $30,000 per delivery of LH2 compared to $6,300 for compressed gaseous hydrogen.
enable operation at higher pressures (e.g., 500 bar or higher). Steel tube trailers are most commonly employed and carry approximately 380 kg onboard; their carrying capacity is limited by the weight of the steel tubes. Recently, composite storage vessels have been developed that have capacities of 560–900 kg of hydrogen per trailer. These Chart LH2 tractor trailer tankers have a gross capacity of 17,600 gal / 66,623 liters, a maximum permissible weight of Liquid at 9,665 lb / 4,384 kg of LH2 and and estimated weight full (Capacity) 62,095 lb / 28,165 kg. It becomes obvious a expansive difference between delivery yields of hydrogen in cryogenic form compared to highly compressed tube trailers at only 350 kilos. The generated revenue of hydrogen to distribution between compressed hydrogen and LH2 tells a realistic story of profitability depending on hypothetical wholesale purchase agreements based on present day retail price at the pump of $16.50, i.e. $30,000 per delivery of LH2 compared to $6,300 for compressed gaseous hydrogen.
